一、The principle by which carbon molecular sieves separate air (N₂ and O₂) is not based on the static equilibrium adsorption of molecular size or polarity, but rather on the difference in diffusion rates.
Oxygen (O₂): Has a smaller kinetic diameter (approximately 0.346 nm). Its diffusion rate within the micropores of the carbon molecular sieve is very fast, allowing it to be rapidly adsorbed into the pore channels.
Nitrogen (N₂): Has a slightly larger kinetic diameter (approximately 0.364 nm). Its diffusion rate within the micropores is much slower than that of oxygen.
Although nitrogen can eventually be adsorbed, the pore size of the carbon molecular sieve is precisely controlled so that the speeds at which the two gases diffuse into the pores differ by an order of magnitude (typically, O₂ diffuses hundreds of times faster than N₂).
二、Process Description (Pressure Swing Adsorption, PSA)
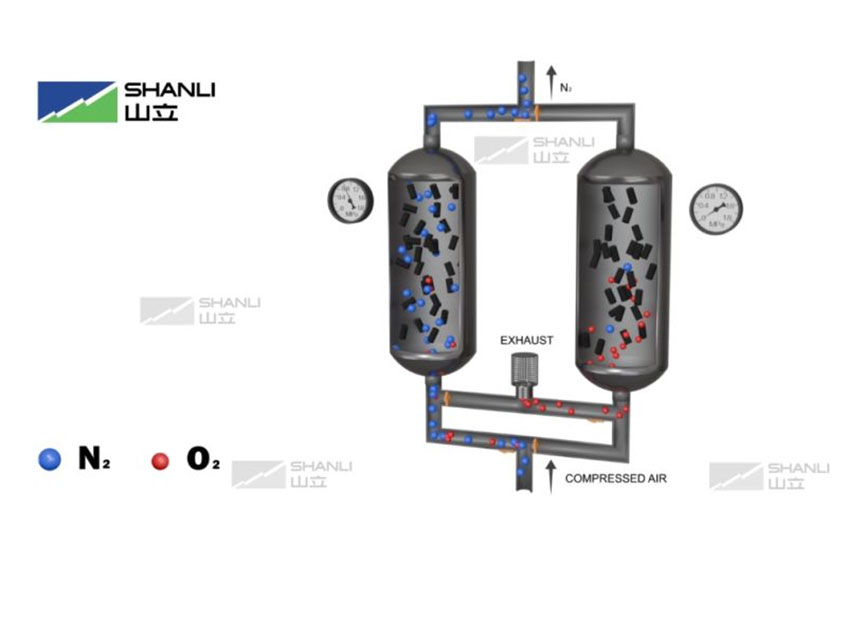
Pressure Swing Adsorption nitrogen generation systems typically consist of two adsorption towers filled with carbon molecular sieve.
Adsorption Stage: Compressed air passes through one adsorption tower from bottom to top. Impurities such as oxygen, water vapor, and carbon dioxide are rapidly adsorbed by the carbon molecular sieve, while the slowly diffusing nitrogen mostly passes through the tower and is output as a high-purity product gas.
Regeneration Stage: Just before this adsorption tower becomes saturated, the system automatically switches to the other tower for operation. The saturated tower is then regenerated by rapidly depressurizing it (desorption), which releases the adsorbed oxygen and other impurities into the atmosphere.
The two towers alternate between adsorption and regeneration, thereby enabling continuous nitrogen production.
If you want to get more information about us,you can click www.carbon-cms.com.
Copyright @ 2026 Chizhou Shanli Molecular Sieve Co., Ltd. All Rights Reserved.
 Network Supported
Network Supported
